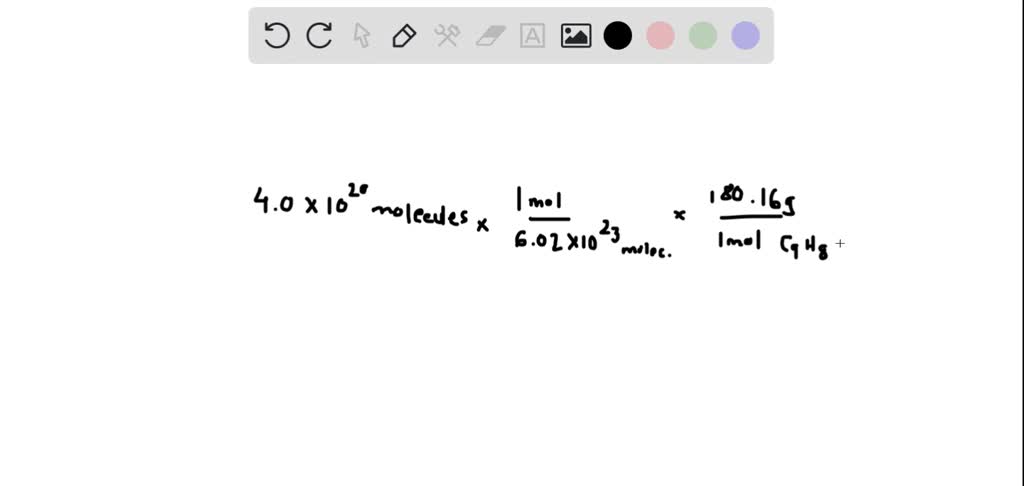
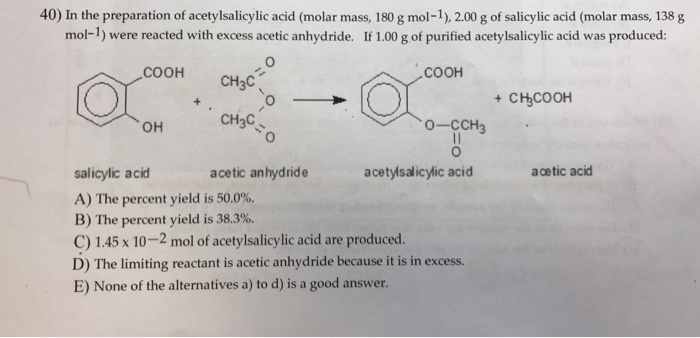
If 5.00 g of acetic anhydride (molar mass = 102.1 g/mol) were completely converted to aspirin (molar mass = 180.08), how many grams of aspirin would you expect to make? | Homework.Study.com
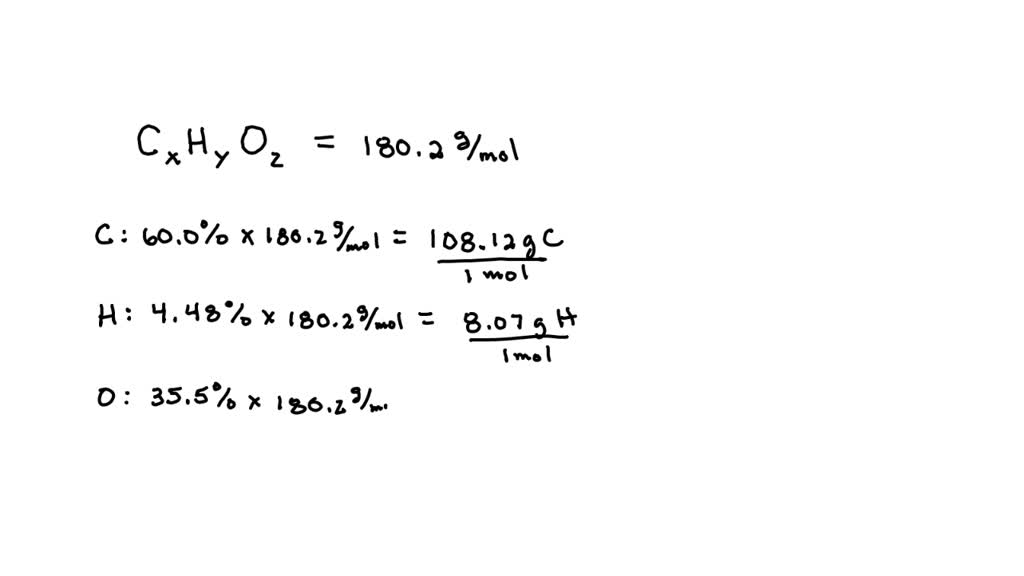
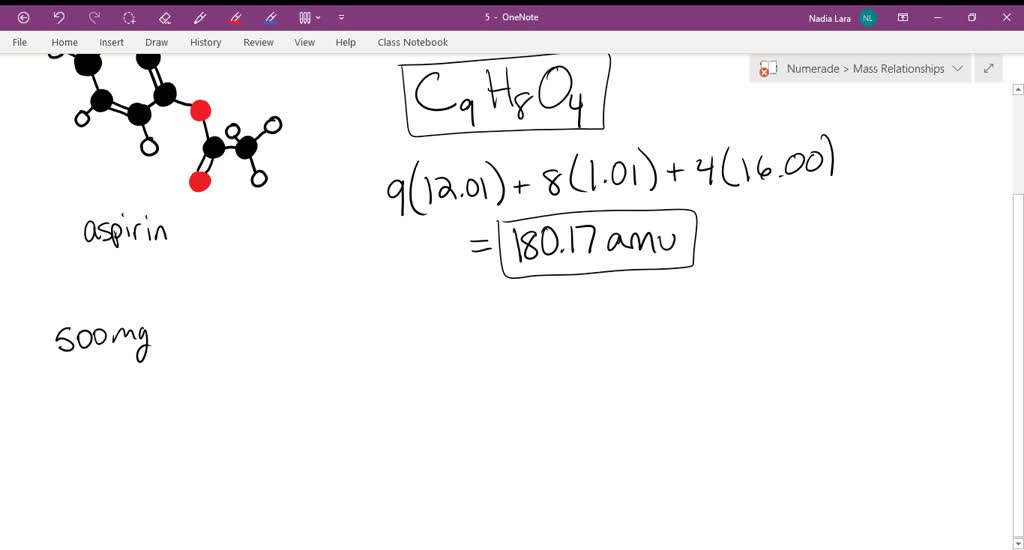
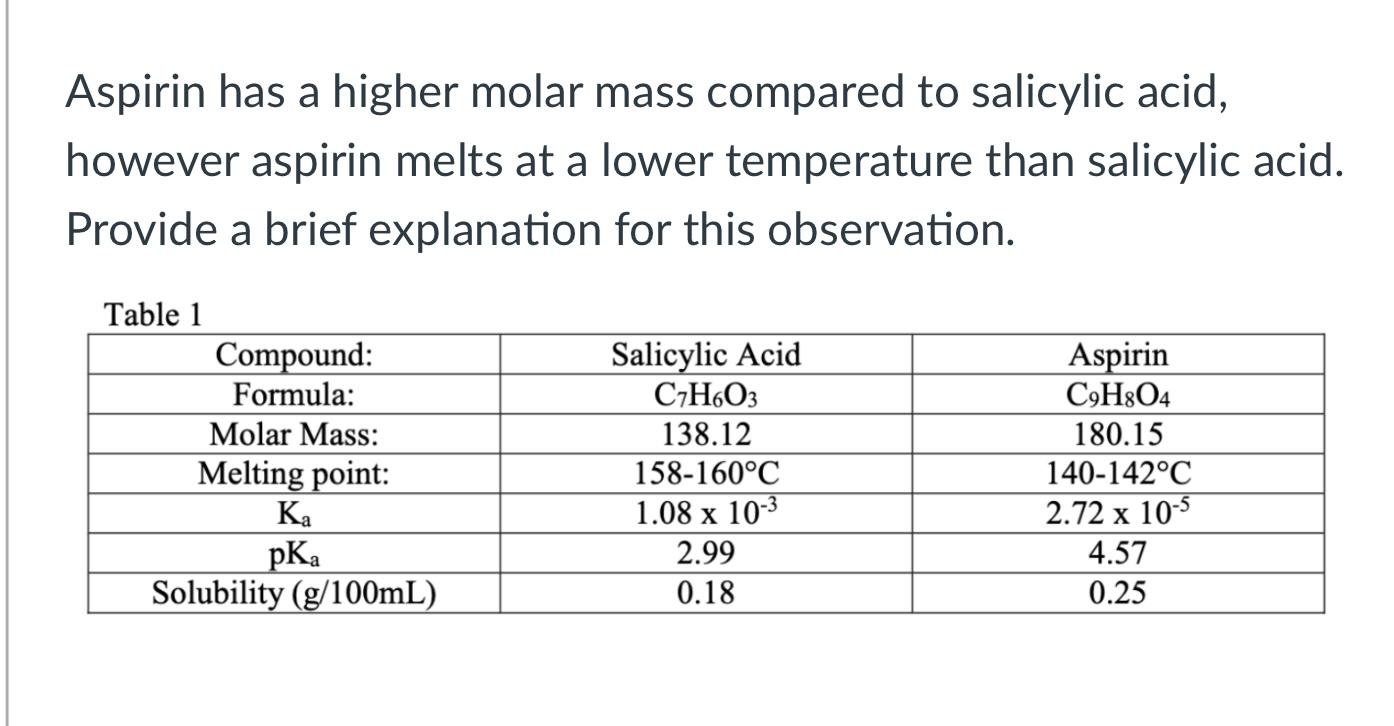
SOLVED: Aspirin has a molar mass of 180.2 g/mol and a composition of 60.0 wt% C, 4.48 wt% H and 35.5 wt% O. What is a molecular formula for aspirin?

Calculate the mass percentage of aspirin (C9H8O4) in acetonitrile (CH3CN) when 6.5g of C9H8O4.... - YouTube
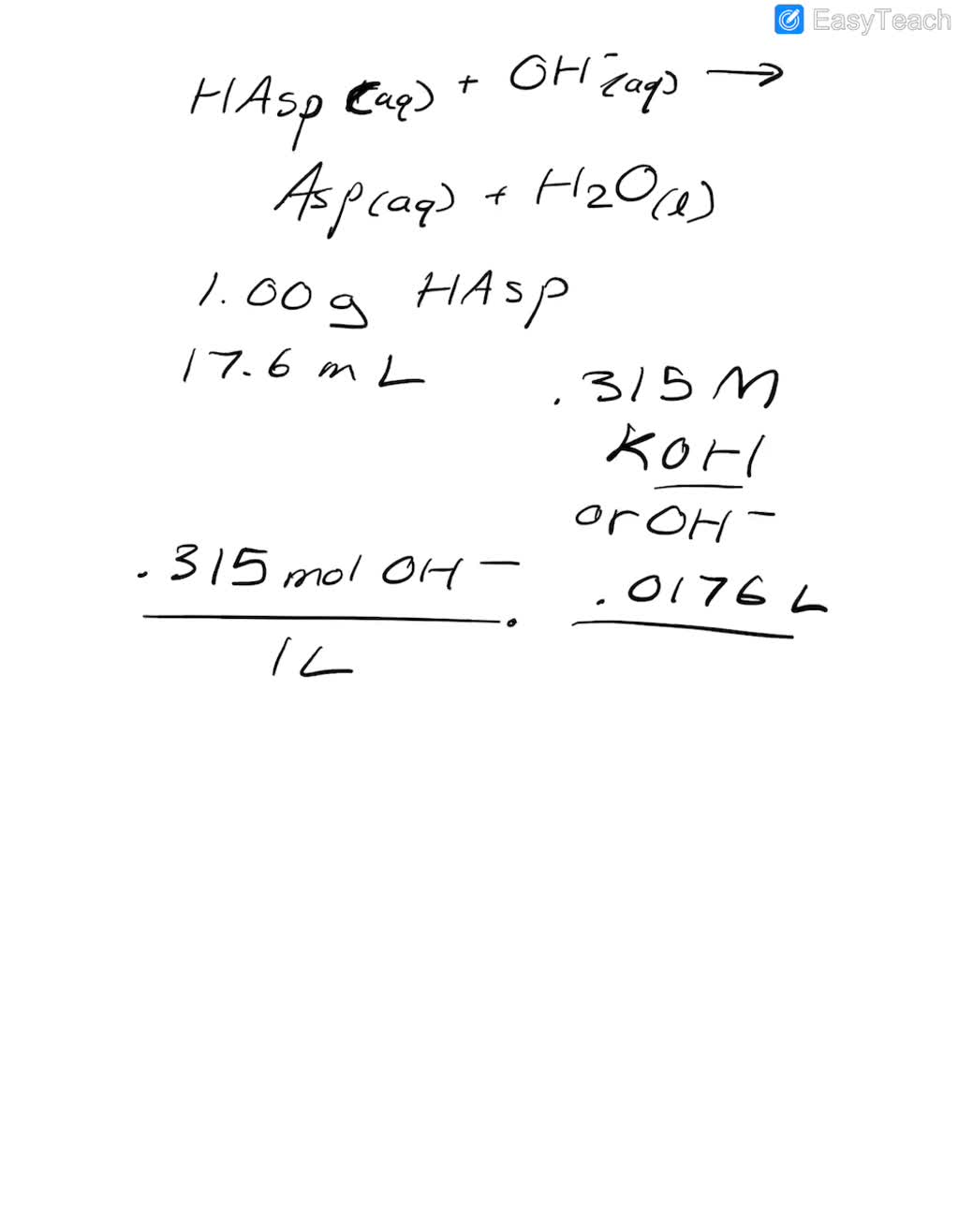
SOLVED: A student tries to determine experimentally the molar mass of aspirin (HAsp). She takes 1.00 g of aspirin, dissolves it in water, and neutralizes it with 17.6 mL of 0.315 M
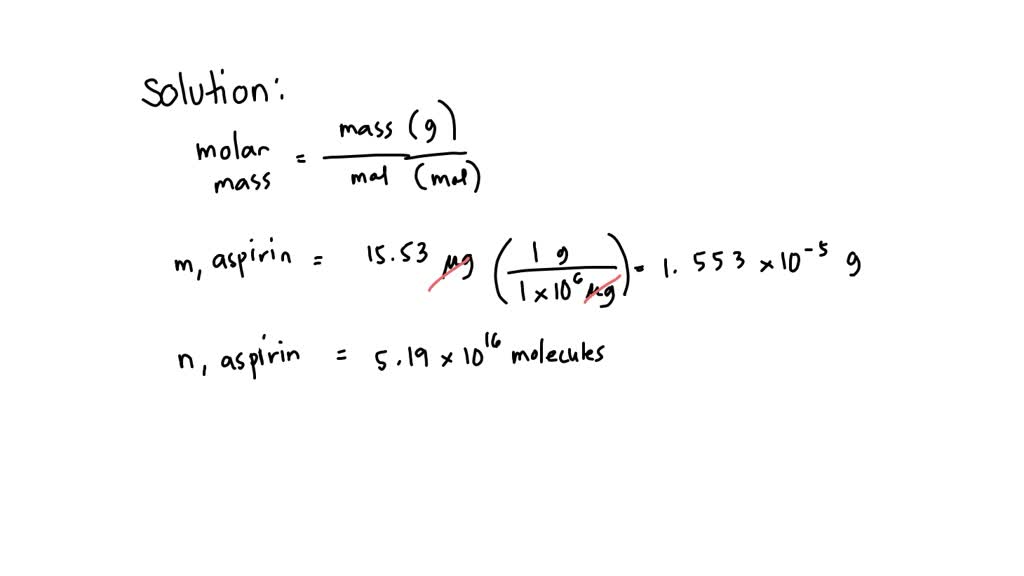
SOLVED: What is the molar mass of aspirin if 5.19 x 10^16 molecules of aspirin weigh 15.53 g? A) 180 g/mol B) 80.6 g/mol C) 133.8 g/mol D) 200 g/mol

Calculate the mass percentage of aspirin (C2H804) in acetonitrile (CH:CN) when 6.5 g of CH8O4 is dissolved in 450 g of CH3CN. (Ans: 1.424)

The molecular formula of acetylsalicylic acid aspirin, one of the most commonly used pain reliever - YouTube